China's first successful synthesis of graphene
Led Color String Light,Twinkly Led String Light,Colorful Led String Lights,Led String Lights Outdoor Tianjin Jinji Optoelectronic Technology Co., Ltd. , https://www.tjjjgd.com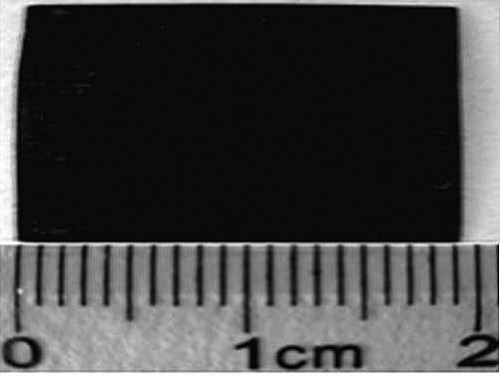

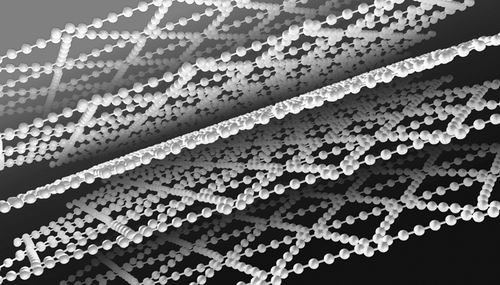
In recent years, Li Yuliang, a researcher at the Institute of Organic Solids, Institute of Chemistry, Chinese Academy of Sciences, led a team to synthesize a two-dimensional carbon with intrinsic band gap sp hybridization on a copper surface from a new perspective of surface chemical reaction combined with solid-state growth and synthesis chemistry. The new allotrope graphene alkyne opens up a precedent for the artificial chemical synthesis of carbon allotropes.
First chemical synthesis
In the 1990s, the Institute of Chemistry All Solid State Laboratory of the Chinese Academy of Sciences undertook the research on carbon material fullerene under the leadership of Zhu Daoben, an academician of the Chinese Academy of Sciences.
According to reports, carbon has three hybrid states of sp3, sp2 and sp, and various carbon isoforms can be formed by different hybrid states. For example, diamond can be formed by sp3 hybridization, and carbon nanotubes, fullerenes, graphene, and the like can be formed by hybridization of sp3 and sp2.
Because of the linear structure, no cis-trans isomers and high conjugation of carbon-carbon triple bonds with sp hybridization, scientists have been eager to obtain new allotropes of carbon with sp hybridization state, and believe that Carbon-based materials have excellent electrical, optical, and optoelectronic properties and will be key materials for the next generation of new electronic and optoelectronic devices.
In 2004, researchers at the University of Manchester in the United Kingdom used a layer of graphite tape on a piece of scotch tape to obtain a graphene of carbon atom thickness. Subsequently, they found that the single-layer graphene had high hardness and good toughness, which was the best known conductive material at that time. The electron mobility of up to 15000 cm2·V-1·S-1 at room temperature makes graphene the hope of manufacturing high-speed transistors.
In 2010, single-layer graphene has gradually moved from the laboratory to the industrialization road, and the basic work of British scientists also won the Nobel Prize in Physics.
Just this year, chemists at the Chemical Solid State Laboratory of the Chinese Academy of Sciences created a chemical synthesis method to create another new carbon material, graphene. The researchers used a coupling reaction of hexadecanylbenzene on the surface of the copper sheet to successfully synthesize a graphene film on the surface of the copper sheet.
This is the first time in the world that chemically obtained all-carbon materials, opening up the precedent for artificially chemically synthesizing carbon allotropes, which has encouraged chemists.
Intensive research
In the past two years, the research institute of Graphene and alkyne of the Institute of Chemistry of the Chinese Academy of Sciences has continued to carry out the basic and applied research of graphene, and realized large-area and large-scale preparation. At the same time, it has led many international scientists to actively participate in research in this field and promote carbon science. Development and bring rare opportunities for carbon materials research.
The researchers worked with a number of domestic and foreign scientists to find that they have excellent properties and properties in catalysis, fuel cells, lithium-ion batteries, capacitors, solar cells, and mechanical properties.
For example, the researchers realized that the thickness of the graphene film was controllable. For the first time, the layer spacing of the graphene film was 0.365 nm, and the thickness of the few layers of graphene film obtained could be controlled between 15 and 500 nm. At the same time, the graphene film exhibits good semiconductor properties and it is found that the conductivity increases with the decrease of the thickness of the graphene. The researchers first determined the hole mobility of the graphene film, and proved the high mobility proposed by the theoretical calculation. The mobility decreases with the increase of the thickness of the graphene film, and the mobility of the graphene film with a thickness of 22 nm can reach. 100~500 cm2·V-1·S-1.
In 2014, the researchers found that graphene-alkyne films are a class of lithium-ion battery anode materials with excellent performance. Graphene alkyne-based lithium-ion batteries also have excellent rate performance, high power, high current, long-term cycle stability, etc. due to their two-dimensional triangular voids of sp and sp2, large surface area, and rapid diffusion of electrolyte ions. Characteristics, related indicators are significantly higher than carbon materials such as graphite, carbon nanotubes and graphene, and have excellent stability. For example, at a current density of 2A·g-1, after 1000 cycles, the specific capacity is still as high as 420 mAh·g-1, which is an advantage that most lithium ion anode materials do not have.
In 2015, the researchers incorporated graphene alkyne into the electron transport layer of the hybrid perovskite device, effectively improving the conductance of the electron transport layer, thereby improving the device performance of the perovskite battery.
The researchers introduced that the introduction of graphene alkyne not only improved the film morphology of the interface material, but also better controlled the interface characteristics, improved the short-circuit current value of the device, thereby increasing the photoelectric conversion efficiency of the device, and the device efficiency was not affected by the voltage scanning conditions. . In addition, the study also found that graphene and P3HT as a modified material for the construction of perovskite solar cells, the photoelectric conversion efficiency increased by 20%. In the opinion of the industry, the above series of research provides new ideas for improving the performance of perovskite batteries and the application development of new carbon materials and the research of perovskite battery devices.
In 2015, researchers conducted research on the performance of graphene-based capacitors and found that they have excellent capacitor performance and capacitance is much higher than other carbon materials. Therefore, the graphite alkyne capacitor can have both high power density and high energy density.
The researchers also found that graphene-supported metal palladium can efficiently catalyze the reduction of 4-nitrophenol, and the reduction rate (0.322 min-1) is 40 times that of Pd-carbon nanotubes, Pd-graphene oxide and commercial Pd carbon, respectively. Double and 5 times; nitrogen-doped graphene has very excellent oxygen reduction catalytic activity, which has been equivalent to commercial platinum/carbon materials, and is expected to replace platinum-based catalysts of noble metals. Because of the high chemical activity of the graphite alkyne triple bond, graphene-based materials such as TiO2(001)-graphene alkyne composites show unique photocatalytic, electrochemical catalysis and catalytic properties.
In addition, as a buffer layer of quantum dot solar cells, graphite alkyne can greatly improve the efficiency of PbS quantum dot solar cells and can significantly reduce the work function, efficiently promote the hole transport ability of quantum dot solar cells, and significantly improve the quantum dot solar cell photovoltaic Conversion efficiency and stability.
At present, researchers in the Chemical Solid State Laboratory of the Chinese Academy of Sciences are still trying to obtain a single-layer structure of graphene by controlling growth and physical stripping methods like graphene. "Although there are difficulties, it has made great progress and it is possible to solve this problem in a short time." Li Yuliang said. For the monomer synthesis of graphene, Li Yuliang believes that its large-scale preparation and industrialization will take time.
Hopeful future
As a new material with China's independent intellectual property rights, the discovery of graphene has an important impact on the international community. It was evaluated by peers as “a remarkable development of carbon chemistry and a real major discoveryâ€.
A study by the famous German physicist Professor Gorling pointed out that graphene is a Dirac cone material, which he believes is an important factor in the band gap of graphene in many properties over zero bandgap graphene.
"Today's Materials" journals titled "Flat-packed carbon" pointed out that "synthesis and separation of new carbon allotropes are the focus of research in the past two or three decades. Chinese scientists first chemically synthesized 3.6 square centimeters of graphene films. Its excellent performance is comparable to silicon, and it may become a key material for future electronic devices together with graphene..."
Markus Zahn, a professor at the Massachusetts Institute of Technology, believes that graphene can have an irreplaceable role in seawater desalination, filtering out 99.7% of sodium chloride in seawater. Internationally renowned scientists have discovered potential applications in the fields of optics, electricity, optoelectronic devices, catalysis, solar cells, etc. through computer simulation, theoretical calculations and experiments.
At present, international and domestic research groups such as the United States, Canada, Japan, Australia, and Germany have conducted research to make graphene research into a period of rapid development.
Not only in academia, but also in the business world, there is a strong interest in the application of graphene. Studies have shown that graphene has great potential applications in energy, catalysis, optics, electricity, optoelectronic devices and many other fields.
The British magazine "Nanotechnology" once listed graphite alkyne with graphene and silene as the most potential and commercial value materials in the future, and made a single chapter of graphite alkyne for market analysis, which is believed to be widely used in many fields. Applications. According to the magazine, the European Union has included research on graphene-related research in the next framework plan, and the United States, Britain and other countries have also included it in its government plan.
The world's two famous business information company research and market and Nissho Global Information Co., Ltd. reviewed the global nanotechnology and materials commercial market before 2019, and considered that graphene is one of the most promising nanomaterials.
Today, graphene is a rising star in the field of carbon chemistry and is widely expected in foundations and applications.
