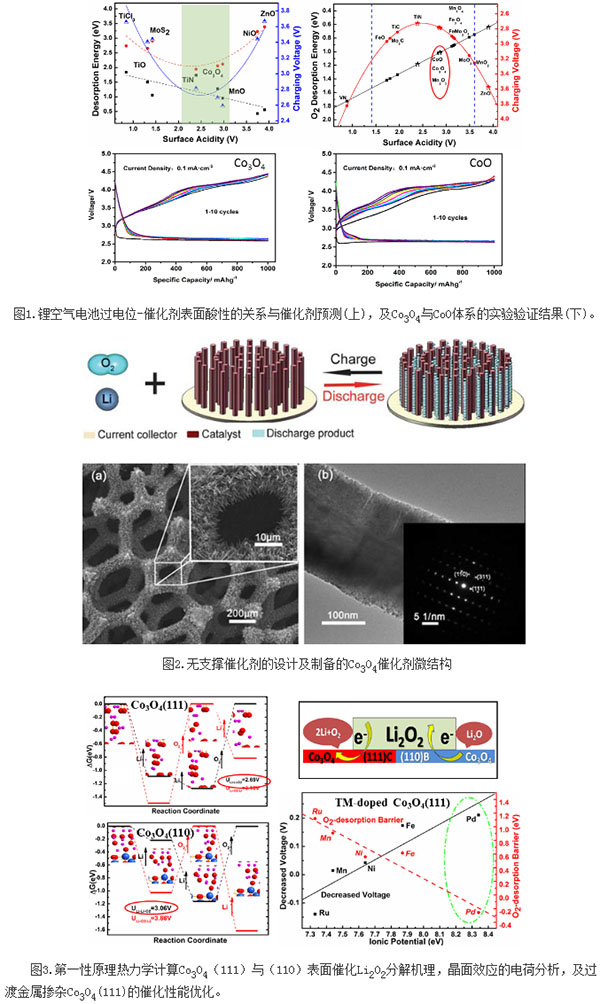
Shanghai Silicate Group made progress in the research field of high-performance lithium-air batteries
The high specific energy lithium-air battery is one of potential power supply technologies for high-capacity pure electric vehicles in the future. However, due to the low rate of charge kinetics, its actual performance is limited, resulting in high overpotential, poor cycling performance, and low current density. Electrode material instability, electrolyte decomposition and other issues. Developing cheap, high-activity catalysts and increasing the reaction rate are the research hotspots for lithium-air batteries. Over the past five years, there have been more than 900 relevant papers, and more than 70 kinds of catalysts have been tried, but the research work lacks design and systematization. The development of material design and calculations and the combination of computation and experimentation are important approaches for the rapid advancement of lithium-air battery research and the development of the “material genomeâ€. Liu Jianjun, a researcher at the Shanghai Institute of Ceramics, Chinese Academy of Sciences, and Wen Zhaoyin cooperated to combine theoretical calculations and electrochemical experiments to perform computational studies on several typical transition metal oxides, carbides, and nitrides, establishing lithium ion desorption and The theoretical model of the relationship between the oxygen evolution barrier and the surface acidity of the catalyst predicts the high activity of the catalyst within the surface acid range of 2.4-3.1 eV. Several highly active catalysts such as Co3O4, CoO, Mo2C, TiC and TiN are proposed. Through the comparative experiments of electrochemical potentials such as overpotential and cycle life of CoO and Co3O4 system, the proposed theoretical model and material system were verified. The research results were published in the Journal of the American Chemical Society (10.1021/jacs.5b07792). Wen Zhaoyin led the research group to design and prepare a Co3O4 unsupported catalyst with preferential growth on the (111) surface, and obtained excellent electrochemical properties such as capacity and polarization. The results of the study were published in Energy & Environmental Science (2011, 4, 4727). The research team led by Liu Jianjun combined the first-principles calculations with thermodynamic analysis to establish a three-phase interface model for O2/Li2O2/Co3O4, revealing the high catalytic activity of the Co3O4(111) surface, further verifying the surface acidity and catalysis. The theoretical prediction of the activity relationship. The research results were published in the American Chemical Society's publication ACS Catalysis (2015, 5, 73). The relevant research work has been supported by the National Natural Science Foundation of China's key and general projects, the Chinese Academy of Sciences' 100-person plan and key deployment. Exit Sign,Exit Light,Exit Signage,Exit Sign With Lights NINGBO JIMING ELECTRIC APPLIANCE CO., LTD. , https://www.feituosafety.com