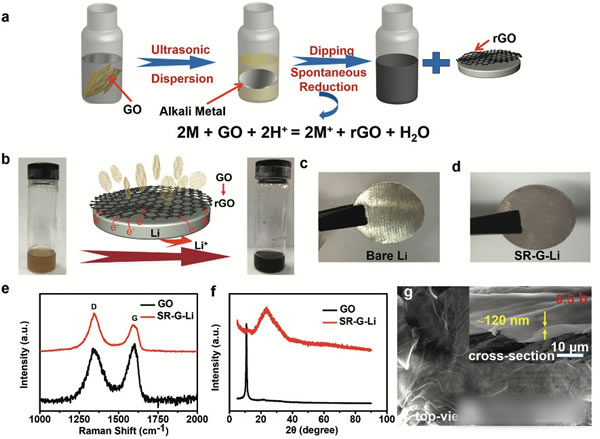
The cycle life is over 1000 times! New type of graphene protection metal lithium negative electrode developed
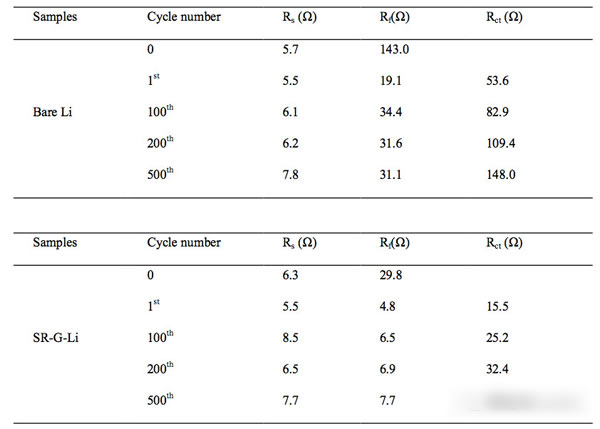
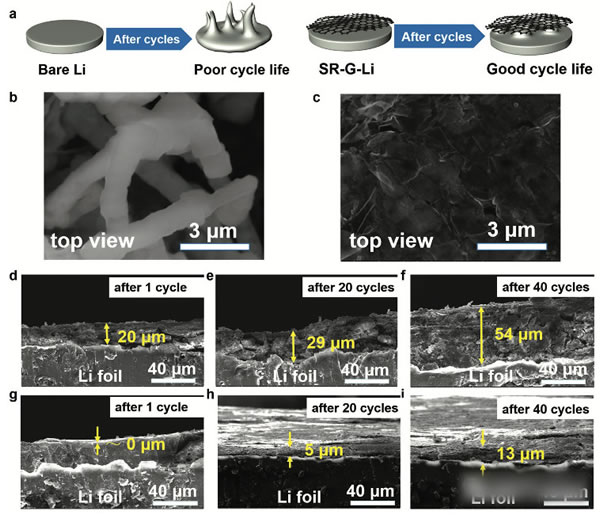
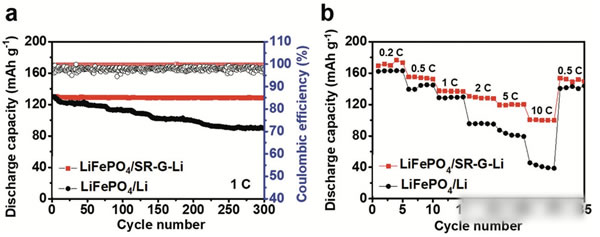
Lithium metal lithium negative is already commonplace, and we have done many articles on metal lithium negatives before. The research on metal lithium negatives can basically be summarized as one sentence “how to avoid the occurrence of lithium dendriteâ€. The advantages of lithium metal negative electrode needless to say, the specific capacity of up to 3860mAh / g, voltage platform -3.05V (vs standard hydrogen electrode), can be said to be a very good selection of negative materials. However, the metal lithium negative electrode also faces a very difficult problem - lithium dendrite, which is a relatively common phenomenon in the electrometallurgy of metals. The growth of dendrites will cause a short circuit in the battery, leading to serious safety accidents. Avoiding the growth of Li dendrites during charging has become a central issue in the study of all metallic lithium negative electrodes. Recently, MaohuiBai (first author) and Keyu Xie (corresponding author) of Northwestern Polytechnical University produced a layer of reduced graphite on the surface of metallic Li by direct reduction of graphene oxide GO using metallic Li. The existence of graphene layer can be very good. A good inhibition of Li dendrite growth, while stabilizing the SEI ink to increase the Coulomb efficiency, the presence of a graphene layer can also significantly improve the rate performance of the metal Li. Experiments have shown that the electrode can be LiPF6 at a current density of 5 mA/cm2. The carbonate electrolyte is circulated 1000 times without short circuit. The figure above shows the preparation process of a graphene coated metal lithium negative electrode. First, the graphene oxide is dispersed in tetrahydrofuran (THF), and then metal lithium is put into the above dispersion, and we can observe the color of the solution gradually. The transition from brown to black indicates that the graphene oxide in the solution gradually transforms the reduced graphene, and the reduced graphene is deposited on the surface of the metal Li to form a protective layer. By controlling the reaction time of the metal Li in the graphene oxide dispersion, the thickness of the graphene layer on the surface of the metal lithium negative electrode can be effectively controlled, thereby obtaining the best electrochemical performance. In order to verify the ability of the metal Li anode prepared in the above process to inhibit the growth of Li dendrites, Maohui Bai prepared two sheets of metallic Li anodes as button cells for cycling tests at different current densities (the results are shown in the figure below). It can be seen from the figure that the voltage fluctuation of the metal Li negative electrode without graphene coating treatment is large during the cycle, and substantially internal short-circuiting occurs in less than 100 times, and the metal treated with the graphene coating layer The negative electrode of Li has a very stable voltage during the cycle, and there is no obvious short-circuit phenomenon when the cycle life exceeds 1000 times. This is the metal Li negative electrode reported to have the longest cycle life in the LiPF6-carbonate electrolyte. From figures d and e below, we compared the voltage platform of the metal Li anode at different current densities and found that when the current density was increased from 1 mA/cm2 to 5 mA/cm2, the metal Li negative voltage platform without graphene coating was from 54.3. The mV has increased significantly to 449.2mV, while the negative electrode of metal Li coated with graphene has only increased from 19.2mV to 79.3mV, indicating that the graphene coating can significantly reduce the polarization of the metal Li anode. The reason why the graphite coating improves the cycling performance of the metal Li anode can be obtained from the EIS analysis. By comparing the EIS results of 1,100,200 and 500 cycles at a current density of 1 mA/cm2, we can find that the graphene coating is The SEI film resistance Rf and the charge exchange resistance RCT of the surface of the metallic Li anode are significantly lower than those without the coating, and the increase in the resistance of the metallic Li anode protected by the graphene coating during the circulation is also significantly slower than that of the ordinary metallic Li anode. It is shown that the graphene coating can help the metal Li anode form a more stable SEI film and slow down the growth of the SEI film during the cycle. After removing the recycled lithium metal negative electrode, the surface of the electrode of the metallic Li negative electrode that is not protected by the graphene coating becomes very rough (lower image b), and many Li dendrites grow. While the surface of the negative electrode of the metallic Li protected by the graphene coating was still very smooth (lower panel c), no significant growth of the metallic Li dendrite was observed. From the side cut surface of the electrode, it can also be seen that the ordinary metal Li negative electrode surface layer becomes very sparse and porous after cycling, and the thickness reaches 54 um after 40 cycles, and the thickness variation reaches 170%. On the other hand, the thickness of the SEI film on the surface of the metal Li negative electrode with graphene protection was only 13 μm after 40 cycles, showing very good stability. In order to verify the practicability of the above electrodes, Maohui Bai also used LiFePO4 as the positive electrode, and metal Li or graphene layer to protect the metal Li as the negative electrode to make a full battery electrochemical test. From the following figure a, we can see that after 300 cycles, we can see The battery capacity of the metal Li anode protected by the coating has almost no decline, while the battery capacity of the common metal Li anode declines to 69% of the initial capacity, indicating that the graphene coating can significantly improve the cycle performance of the metal Li anode. . As can be seen from the magnification test results in figure b below, the discharge capacity of graphene-coated metal Li anode batteries at high magnification is significantly higher than that of ordinary metal Li anodes, indicating that the graphene coating is used to lift the battery. Magnification performance also has obvious help. The graphene coating developed by Maohui Bai protects the Li cathode of the metal and inhibits the growth of the Li dendrite very well, enabling the Li anode to circulate more than 1000 times without short circuit, while the presence of the graphene coating can also improve the metal Li anode. The structural stability of the surface SEI film improves the coulombic efficiency of the battery and improves the capacity retention rate of the battery in the long-term cycle. The most important is that this process has the potential for large-scale application, and the graphene deposition process can be changed to a spray method, thereby greatly increasing the production efficiency and making the technology extremely practical. Magnetic Pump have the magnetic seal, pump no leakage, safety, stable operation. Widely used in chemical industry and food industry. we have Magnetic Gear Pump, magnetic centrifugal pump, magnetic Screw Pump and magnetic rotor pump. base on all kind of pump's properties, pump be installed a magnetic coupling, the conveying medium has no leakage and the working environment is clean and tidy. No need to change the seal, easy to use and easy to operate. Depending on the medium being transported, the working environment is different and different pumps are selected. Magnetic Pump,Magnetic Water Pump,Magdrive Pump,Mag Drive Pump Hengshui Yuanhan Trading Co.,Ltd , https://www.yuanhanpump.com