Chemical oxidation treatment of steel (blue or black)
The chemical oxidation treatment of steel parts refers to the process of placing steel parts in a solution containing an oxidizing agent to form a thin and dense blue-black or dark black film on the surface, also known as the "blue" of steel. Or "black". (3) Pickling. Industrial acid sulfuric acid, hydrochloric acid, phosphoric acid, etc. can be used for pickling. B. Chemical Oxidation The solution of chemical oxidation is generally alkaline. The typical solution ratio and treatment conditions are shown in Table 2-3. The treatment process has two types: single tank method and double tank method. The single-slot method is simple to operate and widely used. The double-slot method is to perform two oxidation treatments on steel in two oxidizing solutions with different concentrations and process conditions. The oxide film obtained by this method has good corrosion resistance and good redness on the metal surface. In the chemical oxidation process, Although the normal temperature blackening process has the characteristics of simple operation and high oxidation speed, there are disadvantages such as unstable performance of the blackening treatment liquid and poor adhesion of the film layer, and it is required to be sealed with a dehydration inhibitor and paraffin to improve corrosion resistance. performance. Machines For Finishing Banners Machines For Finishing Banners,Welding Shop Banner,Banner Edge Welder,Banner Spot Welder NINGBO ZONGLAN MECHANICAL AND ELECTRICAL EQUIPMENT MANUFACTURE CO., LTD , https://www.zonglaneyelet.com
The steel blue-blue process has low cost, high work efficiency, and can maintain the precision of the parts, and is especially suitable for the protection of various mechanical parts that do not allow plating or painting. However, it should be noted that high temperature alkaline oxidation has the risk of causing alkali brittleness. Therefore, the oxidation of steel is often used for the protection of mechanical parts, instruments, instruments, springs, and weapons.
The oxidation treatment can be classified into a high temperature oxidation treatment and a normal temperature oxidation treatment depending on the oxidation treatment temperature. It should be noted here that the temperature of the high temperature oxidation and the normal temperature oxidation treatment are different, the composition of the oxidizing liquid used is also different, the film forming mechanism is different, and the composition of the film is also different.
Oxidation of steel parts is different from natural oxidation under natural conditions or other conditions, but is a surface treatment method that artificially improves the corrosion resistance of steel parts. Its characteristics are:
(1) The oxide film layer is thin and has a thickness of 0.5 to 1.6 μm, which is suitable for steel parts with high precision and dimensional requirements.
(2) The color of the film is generally blue-black or dark black. The color depends mainly on the composition of the steel parts, the surface distribution state, and the oxidation treatment process. In general, the oxide film of steel parts with a high silicon content is grayish brown or dark brown.
(3) The steel parts after oxidation treatment have poor corrosion resistance. If it is saponified by soapy liquid, 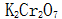 After solution passivation or oil immersion treatment, its resistance to salt spray corrosion increases several to several tens of times.
After solution passivation or oil immersion treatment, its resistance to salt spray corrosion increases several to several tens of times.
2.1.1 High Temperature Chemical Oxidation of Steel Parts High temperature chemical oxidation is a traditional method of blackening. The high-temperature chemical oxidation treatment of steel parts refers to the formation of an oxide film on the surface of steel parts after treatment for a certain period of time (15-90 min) in a concentrated alkaline solution containing an oxidizing agent (such as sodium nitrite) at about 140 °C. process. The film thickness obtained by high temperature chemical oxidation is 0.5-1.5 μm, and the thickest part is only 2.5 μm. The main component is 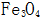 . The film layer has good adsorptivity. The oxide film is immersed in oil or saponified to greatly improve its corrosion resistance. Since the high-temperature oxide film is thin, it has little effect on the size and accuracy of the parts, so it can be directly used after oxidation treatment, and is suitable for precision instruments, optical instruments, weapons, and the like.
. The film layer has good adsorptivity. The oxide film is immersed in oil or saponified to greatly improve its corrosion resistance. Since the high-temperature oxide film is thin, it has little effect on the size and accuracy of the parts, so it can be directly used after oxidation treatment, and is suitable for precision instruments, optical instruments, weapons, and the like.
2.1.1.1 Chemical reaction mechanism After the steel piece enters the solution, the surface is generated by the action of the oxidant and the alkali. 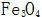 ;, including 3 stages.
;, including 3 stages.
(1) The surface of steel produces sodium ferrite under the action of hot alkali solution and oxidant (sodium nitrite, etc.): 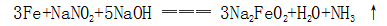
(2) Sodium ferrite is further reacted with an oxidizing agent in the solution to form sodium ferrite: 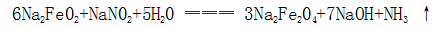
(3) The interaction between sodium ferrite and sodium ferrite forms magnetic iron oxide: 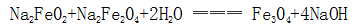
Generated near the steel surface 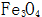 The solubility in the concentrated alkaline solution is extremely small, and it quickly crystallizes out from the solution, and forms a crystal nucleus on the surface of the steel and gradually grows to form a uniform and dense black oxide film.
The solubility in the concentrated alkaline solution is extremely small, and it quickly crystallizes out from the solution, and forms a crystal nucleus on the surface of the steel and gradually grows to form a uniform and dense black oxide film.
2.1.1.2 Electrochemical reaction mechanism After the steel piece enters the electrolyte solution, a galvanic cell is formed on the surface, and iron dissolution occurs in the anode region: 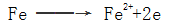
In the presence of strong alkaline conditions in the presence of oxidants, 
At the same time, the hydroxide is reduced at the cathode and dehydrated to form magnetic iron oxide: 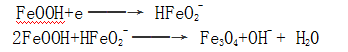
2.1.1.3 High Temperature Oxidation Process The high temperature oxidation process can be divided into three processes: pretreatment, chemical oxidation, and post treatment.
A. Pre-processing The pre-processing mainly includes:
(1) Surface inspection. Mainly to check whether the surface of steel parts has oxide scale, paint film, rust, metal plating, etc. If the above phenomenon exists, it is not suitable for oxidation treatment and should be removed.
(2) Degreasing. The steel parts are placed in a degreasing tank at 90-100 ° C for degreasing treatment, and boiled for 15 to 30 minutes to remove grease from the surface of the workpiece. After the degreasing is finished, the degreaser solution is washed with running water or overflow. Degreasing can be prepared by using a commercially available degreaser. The formula is shown in Table 2-1. 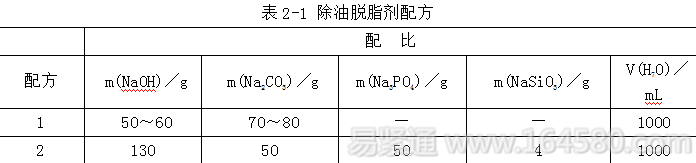
1) Sulfuric acid: It is a strong acid, has good rust removal effect, low volatility, less acid mist, long service life, but high cost. The reaction rate is slow at room temperature, and it is more affected by temperature, which is more likely to produce hydrogen embrittlement. Over corrosion.
2) Hydrochloric acid (HCl): It is a strong acid. It has a fast derusting speed and can be treated at room temperature. It produces hydrogen embrittlement and over-corrosion. It is lighter than sulfuric acid and has low cost, but it has high volatility, poor working conditions and high consumption. It needs to be replaced in time. .
3) Phosphoric acid: It is a medium-strong acid with moderate rust removal effect and non-volatile. After rust removal, a protective phosphating film is formed on the metal surface, which does not cause hydrogen embrittlement and over-corrosion.
Currently, single component acids have rarely been used in the pickling and rust removal of metals. The formulation and process conditions of the steel parts pickling solution are shown in Table 2-2. 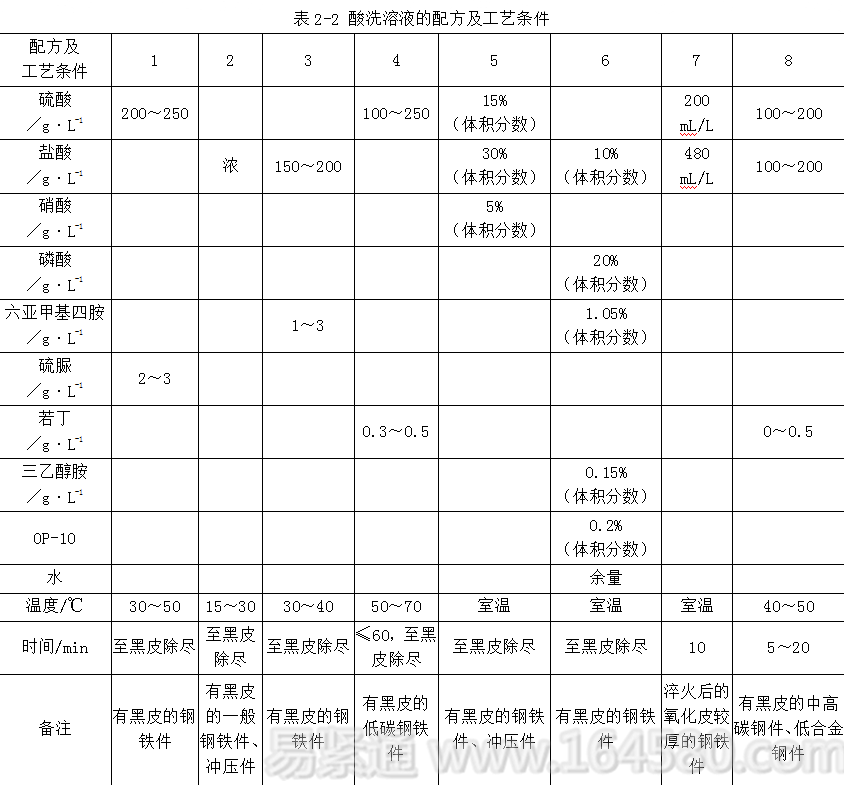
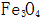 The rate of nucleation and growth on the metal surface directly affects the thickness and quality of the oxide film. First of all,
The rate of nucleation and growth on the metal surface directly affects the thickness and quality of the oxide film. First of all, 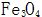 The growth of the crystal nucleus must conform to the law that the total free energy is reduced, otherwise the crystal nucleus will be redissolved.
The growth of the crystal nucleus must conform to the law that the total free energy is reduced, otherwise the crystal nucleus will be redissolved. 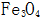 The critical nucleation size depends on its saturation concentration under different conditions.
The critical nucleation size depends on its saturation concentration under different conditions. 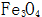 The larger the supersaturation, the smaller the size of the critical nucleus, the large number of crystal nuclei that can grow up, the nucleus grows into crystal grains and quickly meet each other, so that the oxide film formed is relatively fine, but the thickness is relatively thin. on the contrary,
The larger the supersaturation, the smaller the size of the critical nucleus, the large number of crystal nuclei that can grow up, the nucleus grows into crystal grains and quickly meet each other, so that the oxide film formed is relatively fine, but the thickness is relatively thin. on the contrary, 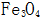 The smaller the supersaturation, the larger the critical nucleus size, the smaller the number of crystal grains per unit area, the larger the oxide film, but the thicker the film. Therefore, all can accelerate the formation
The smaller the supersaturation, the larger the critical nucleus size, the smaller the number of crystal grains per unit area, the larger the oxide film, but the thicker the film. Therefore, all can accelerate the formation 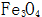 Factors will reduce the grain size and film thickness, but slow down
Factors will reduce the grain size and film thickness, but slow down 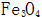 The formation speed factor can increase the grain size and film thickness, so proper control
The formation speed factor can increase the grain size and film thickness, so proper control  The rate of formation is the key to the quality of steel chemical oxidation.
The rate of formation is the key to the quality of steel chemical oxidation.
C. Post-treatment After the chemical oxidation is completed, the steel parts should be cleaned, saponified, and immersed in oil.
(1) Cleaning. First wash with cold running water or overflow, then clean with hot water to remove the alkaline solution on the surface of the workpiece.
(2) Saponification. The saponification of the steel parts is mainly to convert the iron in the pores of the oxide film layer into iron stearate to passivate it to enhance the corrosion resistance. The specific method is: 3% to 5% of soapy water is heated to 80-90 ° C, and the chemically oxidized and cleaned steel workpiece is immersed therein for 3 to 5 minutes; or heated with a mixture of 0.2% chromic acid and 0.1% phosphoric acid. To 60-70~C, immerse the chemically oxidized and cleaned steel workpiece in it for 0.5~1min, then wash it with 70~100°C hot water, then dry it or blow it hot air.
(3) Oil immersion. The oil (or spindle oil, transformer oil) is heated to about 105 ° C, and the saponified and dried steel parts are immersed therein for 3 to 5 minutes.
2.1.1.4 Factors Affecting High Temperature Chemical Oxidation During high temperature chemical oxidation, the performance of the oxide film is mainly affected by the following aspects.
(1) The concentration of sodium hydroxide. It is not good if the concentration of sodium hydroxide is too high or too low. When the temperature is too high, the thickness of the oxide film is slightly increased, but red ash, loose or porous defects are liable to occur, and even the oxide film is dissolved. When the temperature is too low, the oxide film is thin, and spots are easily generated, and corrosion resistance is lowered. , poor protection.
(2) The concentration of the oxidant NaNO2. The increase of the concentration of the oxidant can increase the oxidation rate, and the oxidized ventral layer is dense and firm; the concentration of the oxidant is low, and the obtained oxide film is thick, but loose, and the protective ability is poor.
(3) Processing temperature. When the oxidation temperature is too high, the formed oxide film layer is thin, and red ash is easily formed, resulting in deterioration of the quality of the oxide film.
(4) Iron ion content. In chemical oxidation, the iron ion content in the solution is within a certain range to make the film dense and the bond is firm. Excessive iron ion content will reduce the oxidation rate, and the surface of the steel parts after treatment will be prone to red ash. In general, the iron ion content should be kept at 0.5 to 2.0 g/L. If the iron ion content in the oxidizing solution is too high, it should be diluted and precipitated.
The specific approach is that 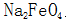 and
and 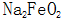 Form of iron oxide
Form of iron oxide 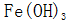 In the form of a precipitate, after filtration, the solution is concentrated by heating and heated to the working temperature to be used.
In the form of a precipitate, after filtration, the solution is concentrated by heating and heated to the working temperature to be used.
(5) Carbon content of steel. In general, an increase in the carbon content of steel leads to an increase in the rate of formation of an oxide film. Therefore, under high temperature chemical oxidation under the same conditions, the oxide film formed by the high carbon steel is thinner than the low carbon steel. Therefore, when the carbon content of the steel piece changes, the temperature and time of the oxidation process should be adjusted. If the carbon content increases, the oxidation temperature should be lowered and the oxidation time should be shortened.
2.1.2 Normal temperature chemical oxidation of steel parts The chemical oxidation of steel parts at room temperature is generally called blackening at room temperature, which is a new technology that has developed rapidly since the 1980s. The normal temperature oxidation of steel parts is consistent with the purpose of high temperature oxidation treatment, but the composition of the solution and the processing conditions of the oxidation treatment are different, and the layers obtained after the treatment are different, not 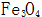 , but CuSe. Compared with high temperature oxidation, blackening at room temperature has the advantages of energy saving, high efficiency, simple operation, low cost, and low environmental pollution.
, but CuSe. Compared with high temperature oxidation, blackening at room temperature has the advantages of energy saving, high efficiency, simple operation, low cost, and low environmental pollution.
2.1.2.1 Normal Temperature Chemical Oxidation Mechanism So far, the mechanism of blackening at normal temperature is still not mature. But most people think that when immersed in a blackening solution at room temperature, the Fe on the surface of the steel piece is in the solution. 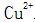 Replaced to allow copper to adhere to the surface of the steel.
Replaced to allow copper to adhere to the surface of the steel. 
Copper reacts with selenite in the solution to form a black copper selenide surface film. 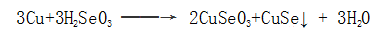
Some researchers believe that the redox reaction of selenite and iron occurs on the surface of steel parts, and a black oxide film of CuSe is formed. 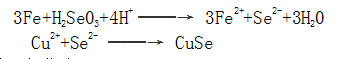
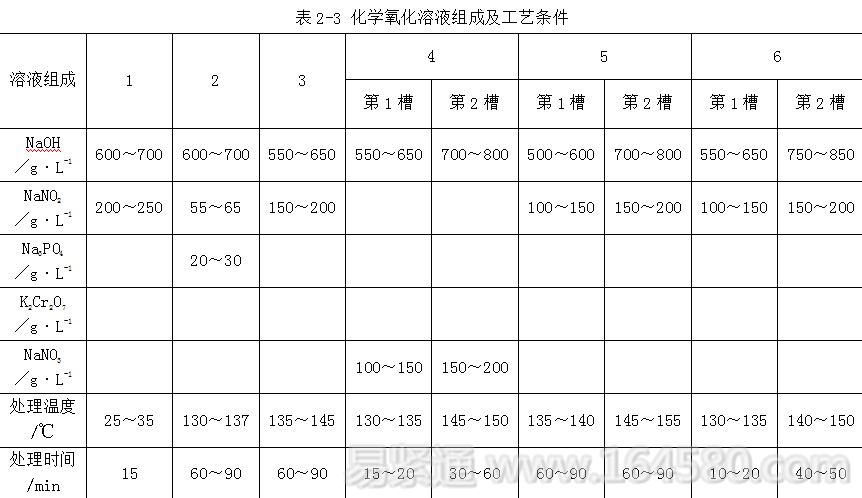
2.1.2.2 Normal temperature oxidizing solution The formulation and process conditions of the normal temperature oxidizing solution are shown in Table 2-4. 
2.1.2.3 Normal temperature oxidation treatment process The normal temperature oxidation treatment process is:
Degreasing→water washing→acid washing→water washing→normal temperature oxidation→air oxidation→water washing→dehydration→immersion oil.
2.1.2.4 Influencing factors of normal temperature oxidation treatment The normal temperature blackening liquid is mainly composed of film forming agent, pH buffering agent, compounding agent and surface wetting agent. The correct selection and proper ratio of these materials are normal temperature. The key factor of black quality control.
(1) Film former. During the blackening process at room temperature, the black CuSe formed on the steel surface mainly comes from the copper salt and selenite of the film-forming substance. The blackening solution for adding phosphate is called an auxiliary film-forming agent because phosphate participates in the reaction to form a phosphate film. The presence of the auxiliary film-forming agent can greatly improve the adhesion, corrosion resistance and the like of the room temperature oxide film.
(2) pH buffer. The pH at room temperature is generally controlled at 2 to 3. When the pH is too low, the reaction rate is too fast, the film layer is loose, and the adhesion and corrosion resistance are lowered. . When the pH is too high, the reaction rate is slow, the film layer is too strong, but the stability of the solution is lowered, and precipitation tends to occur. During normal temperature oxidation, as the reaction proceeds, the solution 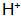 Constantly consumed, the pH will continue to rise. Therefore, the pH buffer is added to keep the pH of the blackening solution within a certain range. Commonly used corrosion inhibitors such as dihydrogen phosphate monophosphate.
Constantly consumed, the pH will continue to rise. Therefore, the pH buffer is added to keep the pH of the blackening solution within a certain range. Commonly used corrosion inhibitors such as dihydrogen phosphate monophosphate.
(3) Stabilizers. Stabilizer in normal temperature blackening liquid is mainly used to match the solution 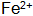 with
with 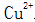 When the steel piece is immersed in the blackening liquid, Fe is oxidized by the action of the oxidizing agent and the acid.
When the steel piece is immersed in the blackening liquid, Fe is oxidized by the action of the oxidizing agent and the acid. 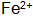 Entering the solution and then further oxidizing it into oxidizing substances and dissolved oxygen in the solution
Entering the solution and then further oxidizing it into oxidizing substances and dissolved oxygen in the solution  . small amount of
. small amount of 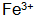 Can be in solution
Can be in solution 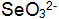 One generation
One generation 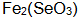 , precipitation, so that the black liquor turbidity failure. When adding stabilizers such as citric acid and ascorbic acid to the blackening liquid, they will
, precipitation, so that the black liquor turbidity failure. When adding stabilizers such as citric acid and ascorbic acid to the blackening liquid, they will 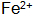 Generate stable complexes and avoid
Generate stable complexes and avoid 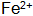 Further oxidation serves to stabilize the solution.
Further oxidation serves to stabilize the solution.
Further, the rate of formation of the surface film greatly affects the corrosion resistance, adhesion, density, and the like of the black film. Too fast a blackening will cause the film to become loose, which will reduce the adhesion and corrosion resistance. Therefore, the addition of citric acid, tartrate, hydroquinone, etc.  Forming complexes, effectively reducing
Forming complexes, effectively reducing 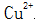 The concentration of the reaction is controlled to increase the film formation time to about 10 minutes. Such complexing agents are also known as speed modifiers.
The concentration of the reaction is controlled to increase the film formation time to about 10 minutes. Such complexing agents are also known as speed modifiers.
(4) Surface wetting agent. The addition of a surface wetting agent can reduce the surface tension of the blackening liquid, making the liquid easily wet on the surface of the steel to obtain a uniform oxide film. In general, the surface wetting agent is a surfactant, and sodium dodecyl sulfate and OP-10 are commonly used. The amount of surface wetting agent is small, accounting for about 1% of the total mass of blackening liquid.
