Ningbo Materials Institute has made progress in the research of inorganic perovskite batteries
Perovskite materials are favored by optoelectronic researchers due to their excellent photoelectric properties and low-temperature and inexpensive preparation methods, especially as an active layer that plays an important role in the field of photovoltaic research. After years of development, its single-junction cell certification efficiency is close to that of monocrystalline silicon. However, the long-term stability of the cell in the working environment limits the commercialization of perovskite cells. In order to solve the perovskite stability problem, Fang Junfeng, a researcher at the Ningbo Institute of Materials Technology and Engineering, Chinese Academy of Sciences, previously used additive engineering (Nat. Commun. 2018, 9, 3806.; J. Mater. Chem. A 2019, 7, 8978; Nano Energy 2019, 58, 825; Adv. Energy Mater. 2019, 9, 1901852; Nano Energy 2019, 64, 103962; Chem. Mater. 2019, 31, 9032) and interface engineering (J. Mater. Chem. A 2020, 8 , 6546; J. Mater. Chem. A 2019, 7, 18898; J. Mater. Chem. A 2019, 7, 3336; J. Mater. Chem. A 2020, 8, 6517) to improve organic-inorganic hybrid calcium Long-term stability of titanium ore batteries. The volatilization of organic components in organic-inorganic hybrid perovskite leads to its photothermal instability. In comparison, all-inorganic perovskite has good temperature resistance and light stability, and inorganic perovskite has the development of perovskite battery One of the important points is the ideal active layer material for the front stage of the high-efficiency laminated structure, but the inorganic perovskite is difficult to control the crystallinity, which limits the improvement of its efficiency. In addition, although inorganic perovskite has good light and heat stability, it is sensitive to water and easily decomposes or undergoes phase change under the condition of a small amount of water, leading to degradation or inactivation of battery performance. Recently, Fang Junfeng has conducted research and made a series of progress around the efficiency limitation and stability of inorganic perovskite cells. Researchers analyzed the device structure and photovoltaic parameters and found that the main factor limiting the efficiency improvement is the open voltage (Voc) loss caused by poor crystallinity and non-radiative recombination. To solve this problem, small organic molecules (trimethylolpropane triacrylate, TMTA) are introduced into the CsPbI2Br perovskite precursor solution. The ester group in the TMTA molecule can effectively improve the crystallization of inorganic perovskite. After high temperature annealing, the double bonds in the molecule are cross-linked. A network is formed to fill the grain boundaries to achieve growth control and in-situ passivation protection; select Thiophene ethylamine iodide (Th-NI) as a post-treatment agent to further inhibit interface recombination and achieve double passivation protection. The device efficiency is improved from 12.17% To 15.58%, and the unencapsulated device showed excellent humidity stability, stored for 1540 h under the relative humidity (RH) 25%, still maintaining 83.4% of the initial efficiency (ACS Energy Lett. 2020, 5, 676-684) . The unstable dangling bonds (defects) distributed on the surface of the perovskite are another factor that limits the stability and efficiency of photovoltaic devices. Defects provide channels for external damage to promote the decomposition and phase change of the perovskite, and act as a recombination center to limit carriers Transmission causes surface recombination and photovoltaic losses, especially for the backward efficiency development of reverse structure inorganic perovskites. In response to this problem, the researchers used bromoformamidine salt (FABr) for surface treatment. Under annealing at 150°C, the diffusion of FABr induces an additional built-in electric field in the perovskite to accelerate charge separation, while giving the inorganic perovskite effective Surface defect passivation and Br-rich protection, Voc increased from 1.078V to 1.223V, achieving the highest reported efficiency of the reverse CsPbI2Br battery 15.92%, and the device kept 91.7% when stored at RH 20% humidity for 1300 h (Nano-Micro Lett. 2020 , 12, 1-13). CsPbI3 perovskite has a more suitable bandwidth, however, phase transition and thin film non-dense problems are its weaknesses. In response to this problem, methylamine picolinate (MAPyA) is used to repair and passivate the surface in situ. MAPyA is decomposed into methylamine gas and picolinate ions when annealed at 100°C. Methylamine gas can repair non-dense film shapes. Appearance, pyridine carboxylate can effectively passivate the grain boundary and surface, and its strong interaction can inhibit the phase change of CsPbI3. At the same time, the oriented pyridine carboxylate can isolate water molecules from damage and solve the problem of humidity phase change and film compaction. The device achieves an efficiency of 16.67%, which is the highest reported reverse inorganic perovskite efficiency, and the unencapsulated device maintains an initial efficiency of 81.13% at a maximum power output of 1800min in the RH 30% air, achieving an unencapsulated inorganic perovskite in the air The long-term output of the device (ACS Energy Lett. 2020, 5, 3314-3321). The research work is supported by the National Natural Science Foundation of China, the Natural Science Foundation of Zhejiang Province, the Frontier Science Key Research Program of the Chinese Academy of Sciences, and the National High-level Talent Special Support Program. Kitchen Floor Drain,Stainless Steel Kitchen Floor Drain,Hidden Square Gold Shower Floor Drain,Round Black Shower Floor Drain Kaiping City Jinqiang Hardware Products Co.,Ltd , https://www.jmkimpowerdrain.com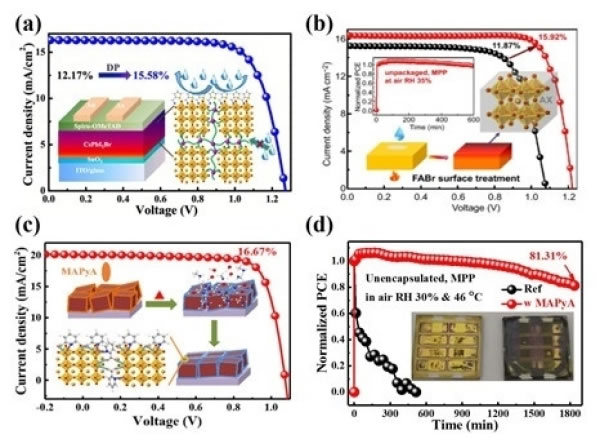
(A) Schematic diagram of double passivation; (b) Schematic diagram of high-temperature FABr modification; (c) Schematic diagram of in-situ repair; (d) Comparison of air MPP performance of in-situ repair devices
